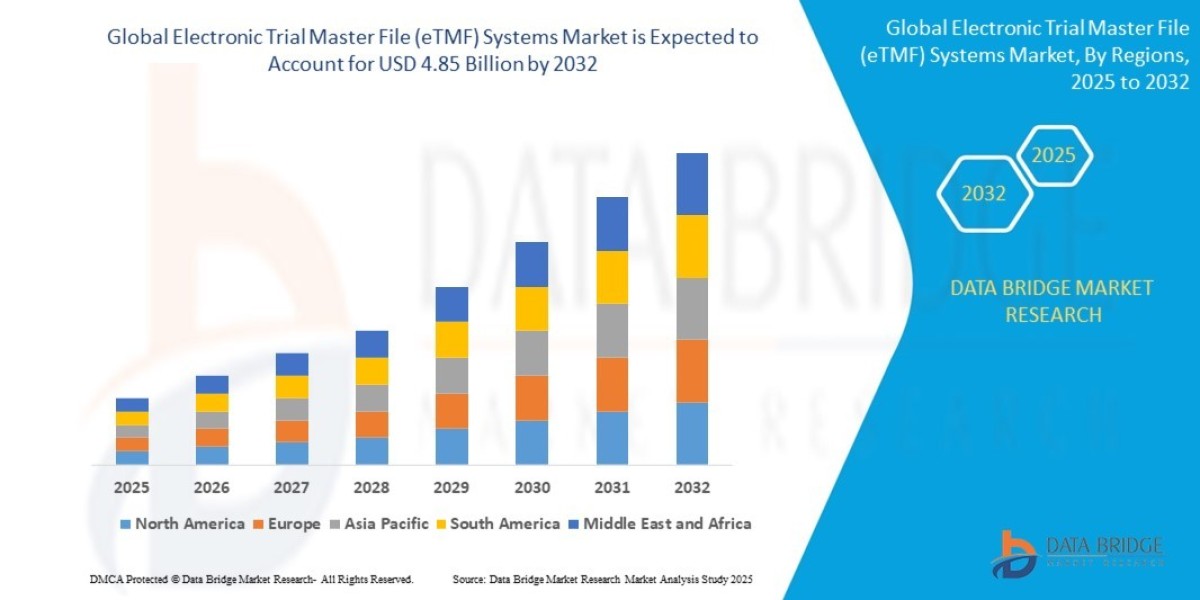
"Executive Summary Electronic Trial Master File (eTMF) Systems Market :
- The global electronic trial master file (eTMF) systems market size was valued at USD 1.84 billion in 2024 and is expected to reach USD 4.85 billion by 2032, at a CAGR of 12.90% during the forecast period

Electronic Trial Master File (eTMF) Systems Market research report is the comprehensive analysis on the study of industry. Further, manufacturer can adjust production according to the conditions of demand which are analysed here. Analysis and discussion of important industry trends, market size, and market share estimates are revealed in the report. Additionally, the report helps the manufacturer in finding out the effectiveness of the existing channels of distribution, advertising programmes or media, selling methods and the best way of distributing the goods to the eventual consumers. The world class Electronic Trial Master File (eTMF) Systems Market report also supports to secure economies in the distribution of products and find out the best way of approaching the potential.
By understanding and keeping into focus customer requirement, one method or combination of many steps have been employed to structure the most excellent Electronic Trial Master File (eTMF) Systems Market research report. The report is generated with the systematic gathering and analysis of information about individuals or organizations which is conducted through social and opinion research. This global market report analyses key factors of the industry which offers precise and accurate data and information for the business growth. What is more, competitive analysis gives a clear idea about the strategies used by the major competitors in the Electronic Trial Master File (eTMF) Systems Market that perks up their penetration in the market.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Electronic Trial Master File (eTMF) Systems Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/global-electronic-trial-master-file-etmf-systems-market
Electronic Trial Master File (eTMF) Systems Market Overview
**Segments**
- Based on component, the global electronic trial master file (eTMF) systems market can be segmented into software and services. The software segment is expected to dominate the market due to the increasing adoption of eTMF systems by pharmaceutical companies and contract research organizations (CROs) to streamline clinical trial processes and ensure regulatory compliance. On the other hand, the services segment is anticipated to witness significant growth as organizations seek external expertise to implement and maintain eTMF systems effectively.
- By delivery mode, the market can be categorized into web-hosted eTMF and licensed eTMF. The web-hosted eTMF segment is projected to hold a larger market share as it offers flexibility, scalability, and cost-effectiveness to users. However, the licensed eTMF segment is also gaining traction among organizations looking for greater control and customization options over their eTMF systems.
- On the basis of end-user, the market is divided into pharmaceutical companies, CROs, and others. The pharmaceutical companies segment is expected to lead the market owing to the increasing number of clinical trials conducted by pharmaceutical manufacturers to bring new drugs to market. CROs are also significant end-users of eTMF systems as they play a vital role in managing clinical trial documentation for multiple sponsors.
**Market Players**
- Some of the key players operating in the global electronic trial master file (eTMF) systems market include Veeva Systems, Oracle Corporation, Paragon Solutions, Phlexglobal Limited, TransPerfect, Aurea Software, Mayo Clinic, CareLex, Montrium, and Wingspan Technology. These companies are focusing on strategic partnerships, product innovations, and acquisitions to strengthen their market position and cater to the evolving needs of the healthcare industry.
- Other prominent players in the market are ArisGlobal, eFileCabinet, MasterControl, SureClinical, Dell EMC, Phlexglobal, TRILOGY Writing & Consulting GmbH, Wingspan, and ZenQMS. These players are investing in research and development activities to enhance their eTMF solutions and gain a competitive edge in the market.
For more insights and detailed market analysis, visit: The electronic trial master file (eTMF) systems market is experiencing robust growth driven by the increasing demand for efficient and compliant clinical trial management solutions across the pharmaceutical and healthcare industries. One of the key trends shaping the market is the shifting focus towards software solutions within the eTMF systems segment. Software providers such as Veeva Systems, Oracle Corporation, and Paragon Solutions are at the forefront of offering innovative solutions that cater to the specific needs of pharmaceutical companies and CROs. These software solutions streamline document management, enhance collaboration among stakeholders, and ensure adherence to regulatory requirements, thereby driving their adoption in the market.
Moreover, the services segment within the eTMF systems market is witnessing significant growth as organizations recognize the importance of external expertise in implementing and maintaining complex eTMF systems. Companies like Phlexglobal Limited, TransPerfect, and CareLex are offering consulting, implementation, and training services to support organizations in optimizing their eTMF processes. This trend is expected to continue as organizations look to leverage specialized knowledge and experience to navigate the evolving landscape of clinical trial regulations and compliance standards.
In terms of delivery mode, the web-hosted eTMF segment is gaining traction due to its benefits in terms of flexibility, scalability, and cost-effectiveness. Cloud-based solutions offered by companies like Aurea Software and Mayo Clinic are enabling users to access their eTMF systems remotely, collaborate in real-time, and scale their storage and processing capabilities as needed. On the other hand, the licensed eTMF segment is appealing to organizations seeking greater control and customization options over their systems. Providers such as Montrium and Wingspan Technology are catering to this demand by offering on-premise solutions tailored to the specific requirements of their clients.
Looking at the end-user landscape, pharmaceutical companies are emerging as the primary adopters of eTMF systems, driven by the need to manage the growing volume of clinical trial documentation and ensure compliance with regulatory standards. CROs are also significant users of eTMF systems, playing a crucial role in facilitating efficient collaboration between sponsors, investigators, and regulatory authorities. As the number of clinical trials continues to rise globally, the demand for advanced eTMF solutions from companies like ArisGlobal, MasterControl, and Dell EMC is expected to grow, further propelling the market expansion.
In conclusion, the global eTMF systems market is characterized by a diverse range of players offering software and services tailored to the unique requirements of pharmaceutical companies, CROs, and other stakeholders in the healthcare industry. With ongoing investments in product development, strategic partnerships, and acquisitions, market players are poised to capitalize on the increasing demand for advanced eTMF solutions and drive innovation in clinical trial management practices.The global electronic trial master file (eTMF) systems market is witnessing significant growth driven by the ever-increasing demand for efficient and compliant clinical trial management solutions within the pharmaceutical and healthcare sectors. Market players such as Veeva Systems, Oracle Corporation, and Paragon Solutions are spearheading the adoption of software solutions tailored to meet the specific needs of pharmaceutical companies and contract research organizations (CROs). These innovative software solutions offered by key players streamline document management, enhance collaboration among stakeholders, and ensure adherence to stringent regulatory requirements, thus contributing to the market's expansion.
Furthermore, the services segment within the eTMF systems market is experiencing substantial growth as organizations acknowledge the value of external expertise in effectively implementing and maintaining complex eTMF systems. Companies like Phlexglobal Limited, TransPerfect, and CareLex are providing consulting, implementation, and training services to assist organizations in optimizing their eTMF processes. This trend is expected to persist as organizations seek specialized knowledge and experience to navigate the evolving landscape of clinical trial regulations and compliance standards effectively.
In terms of the delivery mode, the web-hosted eTMF segment is gaining momentum due to its inherent benefits in terms of flexibility, scalability, and cost-effectiveness. Cloud-based solutions offered by companies like Aurea Software and Mayo Clinic enable users to access their eTMF systems remotely, collaborate in real-time, and scale their storage and processing capabilities as needed. On the other hand, the licensed eTMF segment appeals to organizations looking for greater control and customization options over their systems. Providers such as Montrium and Wingspan Technology cater to this demand by offering on-premise solutions tailored to the specific requirements of their clients.
Regarding the end-user landscape, pharmaceutical companies stand out as the primary adopters of eTMF systems, driven by the imperative to manage the increasing volume of clinical trial documentation and ensure compliance with regulatory standards. CROs also play a vital role in the adoption of eTMF systems, facilitating efficient collaboration between sponsors, investigators, and regulatory authorities. As the global number of clinical trials continues to rise, the demand for advanced eTMF solutions provided by companies like ArisGlobal, MasterControl, and Dell EMC is projected to grow, further propelling market expansion.
In conclusion, the global eTMF systems market is characterized by a diverse range of players offering software and services tailored to the unique requirements of stakeholders in the healthcare industry. With ongoing investments in product development, strategic partnerships, and acquisitions, market players are well-positioned to capitalize on the escalating demand for advanced eTMF solutions and drive innovation in clinical trial management practices.
The Electronic Trial Master File (eTMF) Systems Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/global-electronic-trial-master-file-etmf-systems-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Answers That the Report Acknowledges:
- Market size and growth rate during forecast period
- Key factors driving the Electronic Trial Master File (eTMF) Systems Market
- Key market trends cracking up the growth of the Electronic Trial Master File (eTMF) Systems Market.
- Challenges to market growth
- Key vendors of Electronic Trial Master File (eTMF) Systems Market
- Opportunities and threats faces by the existing vendors in Global Electronic Trial Master File (eTMF) Systems Market
- Trending factors influencing the market in the geographical regions
- Strategic initiatives focusing the leading vendors
- PEST analysis of the market in the five major regions
Browse More Reports:
North America Lung Cancer Surgery Market
North America Dental Implants Market
Global Cold Chain Testing Market
North America Internet of Medical Things (IoMT) Market
Global Neonatal Ventilators Market
Global Décor Paper Market
Global Clinical Workflow Solutions Market
Global Retinal Biologics Market
Global Automotive Electric Scooter Market
Global Baby Cribs and Cots Market
Asia-Pacific Coordinate Measuring Machine Market
Global Cloud Field Service Management Market
Global Bike Tyre Market
Global Database Encryption Market
Global Alcohol Sensor Market
Global Virtual Visits Market
Global Window Blinds Market
Global Resectoscope Market
Global Lactic Acid for Food Applications Market
Global Non Alcoholic Beverages Market
Europe Bone Marrow Biopsy Market
North America Surgical Instrument Tracking Systems Market
North America Microalgae Market
Global Automotive Mobile Gas Pumping System Market
Global Automotive Tempered Glass Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"